The Trouble With Biofilms
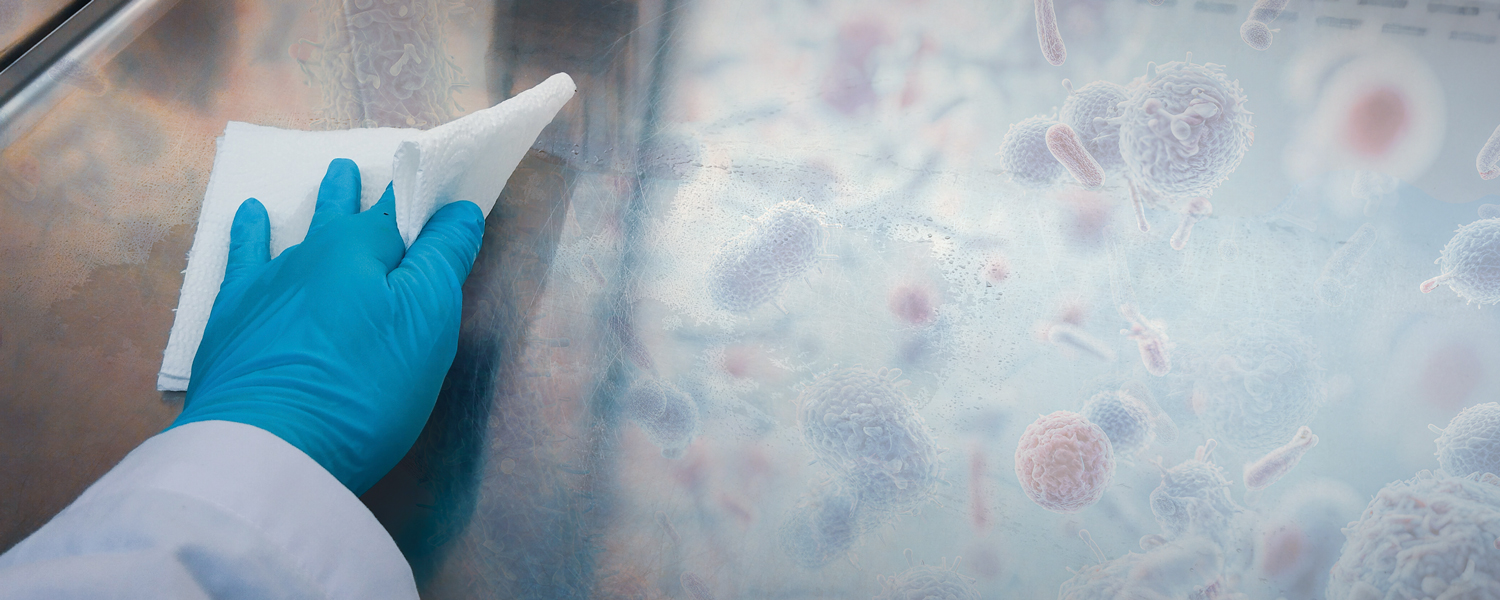
Biofilms are often referred to as “cities of microorganisms” that can adhere to and form on almost any surface. They are increasingly recognized as a critical global health issue across a multitude of industries, with estimated economic costs reaching US$5 trillion annually—the equivalent of $1 million multiplied by 5 million.
Biofilms present a significant challenge in maintaining clean and healthy environments in the built environment, such as schools, hotels, factories, hospitals, stadiums, planes, cars, and boats. It is estimated that between 65-80% of all bacterial and chronic infections originate from biofilms. Biofilms on a surface can cause material degradation, fouling that impedes fluid flow or heat transfer, contamination, infection, and cosmetic discoloration.
Biofilms grow faster and more resistant
Bacteria tend to organize themselves into biofilms on surfaces, where they can develop up to 10 times faster than they would on their own in the environment. The protective layer surrounding them gives them the ability to resist cleaning and disinfection products up to 100 times more effectively. These structured communities of microorganisms adhere to surfaces and are notoriously difficult to remove with conventional cleaning methods.
Even though you may not be familiar with the term “biofilm,” you have undoubtedly encountered them on a regular basis. For example, the plaque that forms on your teeth and causes tooth decay is one type of bacterial biofilm. The “gunk” that clogs your household drains is also a biofilm; a persistent infection from a scrape you got from a sports injury was likely caused by a biofilm, too.
What is a biofilm?
A biofilm is composed of living, reproducing microorganisms that exist as a community.
Biofilms can contain many distinct types of microorganisms, such as bacteria, algae, fungi, yeasts, and protozoa. Biofilms are living communities, and as they mature, many different microbial species will likely develop and coexist with each other. As biofilms grow, they can release cells from the colony, enabling them to spread and colonize other surfaces.
How do biofilms form?
Biofilms can form on both wet and dry surfaces. A biofilm forms when microorganisms attach to the surface of the object and begin to reproduce, protecting each other by secreting a slimy, glue-like substance known as an extracellular polymeric substance (EPS). EPS is primarily composed of polysaccharides, proteins, nucleic acids, and lipids, and it provides structural support, much like the scaffolding around a building with all the gaps filled in.
EPS acts as a protective barrier, making biofilm bacteria and fungi particularly resistant to environmental threats such as cleaning products, disinfectants, and antimicrobials.
Where do biofilms form?
Biofilms can form on just about any surface: floors, walls, fittings, fixtures, furniture, equipment, metals, plastics, natural materials (such as rocks), medical implants, kitchen counters, contact lenses, the walls of a hot tub or swimming pool, human and animal tissue, and much more. Locker room surfaces are prime locations for biofilms.
The extensive surface area of the indoor built environment provides many opportunities for biofilm development.
How big can a biofilm get?
Biofilms can be so thin that they evade detection by the naked eye—sometimes just a few cell layers thick. The biofilms that almost certainly exist on your kitchen counter or your cell phone, for instance, are generally undetectable to the eye.
However, they can also grow to become many inches thick, such as bacteria, fungi, and yeasts in pipes or HVAC systems.
Biofilms fluoresce (If it glows, it goes)
You can detect biofilms by using special ultraviolet (UV) lamps with specific wavelengths that cause a distinctive fluorescence of the cellular and extracellular components of the microorganisms that form the biofilms.
You can also use hand-held devices for rapid-sweeping surveillance of surfaces to detect bacteria. This light reaction allows for direct visual detection of contaminated surfaces, which is essential for assessing the presence of biofilms.
Can you wipe away biofilms?
A report titled “Difficulty in removing biofilm from dry surfaces,” published in December 2019 in the Journal of Hospital Infection, discusses a study conducted by Greg Whiteley and his team and their findings:
- If you dried a culture of bacteria (not a biofilm) onto a stainless-steel surface (which is not a friendly surface for bacterial survival), you could wipe away 99.9% of them with a single swipe of a wet cloth. After five wipes, 99.999% would be removed.
- However, if you took those same bacteria and grew them into a dry surface biofilm, even after 50 wipes, you would only have removed a little over 96%.
- Biofilm bacteria are 100 times harder to remove than bacteria without a biofilm.
How biofilms affect surface cleaning
As stated by Stephanie Dancer in her September 2022 Hygiene article “How Do Biofilms Affect Surface Cleaning in Hospitals?,” this is what we know:
- Eradicating all surface microbes, whether part of a biofilm community or not, will encourage the rapid repopulation of a newly decontaminated surface with environmental, and other, microbes.
- Disrupting wet-surface biofilms following bleach exposure resulted in increased detection of multidrug-resistant organisms, including carbapenemase-producing Enterobacteriaceae.
- Hospitals using disinfectants are more likely to encourage multidrug-resistant pathogens, such as vancomycin-resistant enterococci, Acinetobacter, and gram-negative organisms, such as Stenotrophomonas spp., due to the selection of linked biocidal and antimicrobial resistance characteristics.
- Hospitals that rely on detergent or probiotic-type products encourage the repopulation of surfaces by inert environmental organisms such as Methylobacterium, Bacillus, and other nonpathogens.
The study of biofilms is revolutionary and important
For building managers, facility managers, and cleaning professionals, the study of biofilms is revolutionary and of particular importance. For many years, the effective treatment (i.e., destruction) of harmful microorganisms, such as bacteria, has been approached from the wrong perspective. Harmful microorganisms were studied in isolation as individual organisms, not as members of a biofilm colony, where they usually reside.
New cleaning processes needed
A cleaning product, such as a disinfectant, might be advertised to “kill millions of germs on contact.” While this is a truthful statement from the manufacturer, consider this: those millions of germs are embedded in a biofilm on a surface, held together and protected by an EPS. Because EPS makes up 90% of the biofilm, the germs within the biofilm demonstrate a remarkable resistance to cleaning and disinfecting.
Cleaning products and disinfectants are developed by testing their effects on harmful microorganisms in a planktonic state, where microorganisms float in a solution as individuals, not as part of a biofilm colony attached to a surface. So, while such treatments may indeed kill millions of germs on contact, their effect is severely limited when applied to real-world environments where the microorganisms to be killed are members of a biofilm.
For example, what we know about biofilms is:
- Bacillus subtilis, a strong EPS producer, has been shown to be resistant to chlorine dioxide (0.03%), hydrogen peroxide (7.5%), and peracetic acid (2.25%), and protects Staphylococcus aureus from peracetic acid (0.35%) when in a biofilm.
- Acinetobacter johnsonii was shown to protect the Salmonella enterica subspecies enterica serovar Liverpool in a dual biofilm against benzalkonium chloride (300 mg/L).
New business opportunities as a biofilm buster
The study of biofilms represents a revolutionary way to understand the microbiology of nearly everything around us, from problems that afflict industry to serious public health issues. With this new understanding comes an exciting opportunity for new business—a chance to rethink our strategies for dealing with biofilm problems and to create new solutions that were previously overlooked or not handled correctly because the role of biofilms was not properly recognized and appreciated.
Dr. Gavin Macgregor-Skinner is the senior director of the Global Biorisk Advisory Council® (GBAC), a division of ISSA. As an infection prevention expert and university professor, he works to develop protocols and education for the global cleaning industry to empower facilities, businesses, and cleaning professionals to create safe and healthy environments.
References:
Dancer, S.J. “How Do Biofilms Affect Surface Cleaning in Hospitals?” Hygiene, vol. 2, 2022, pp. 132-135.
Dartnell, L.R., Roberts, T.A., Moore G., Ward, J.M., and Muller, J.P. “Fluorescence Characterization of Clinically Important Bacteria.” PLoS ONE, vol. 8, no 9, 2013, e75270.
Jamal, M., Ahmad, W., Andleeb S., Jalil, F., Imran, M., Nawaz, M.A., Hussain, T., Ali, M., Rafiq. M., and Kamil, M.A. “Bacterial Biofilm and Associated Infections.” Journal of the Chinese Medical Association, vol. 81, 2018, pp. 7-11.
Ledwoch, K., Dancer, S.J., Otter, J.A., Kerr, K., Roposte, D., Rushton, L., Weiser, R., Mahenthiralingam, E., Muir, D.D., and Maillard, J.Y. “Beware Biofilm! Dry Biofilms Containing Bacterial Pathogens on Multiple Healthcare Surfaces; A Multi-centre Study.” Journal of Hospital Infections, vol. 100, Nov. 2018, pp. e47-e56.
Parvin, F., Hu, H., Whiteley, G.S., Glasbey, T., and Vickery, K. “Difficulty in Removing Biofilm from Dry Surfaces. Journal of Hospital Infections, vol. 103, no. 4, Dec. 2019, pp. 465-467.
Sidebar

BONUS VIDEO CONTENT: issa.com/biofilms